Seznamy Aluminium Atom Structure
Seznamy Aluminium Atom Structure. Aluminium visually resembles silver, both in its color and. Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13.
Nejchladnější Aluminum Al American Elements
The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Turn off atom manipulation off; Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom.Following exposure to fresh air, aluminium oxide was cleared.
Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group. One is a deformed octahedron of 6 al atoms at a distance of 217 pm. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. The pudding had positive charge and the electrons having negative charge were like plums on the pudding.

Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2.. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Increase charge of selected atom +1; The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth.

Following exposure to fresh air, aluminium oxide was cleared. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement.

Following exposure to fresh air, aluminium oxide was cleared. Increase charge of selected atom +1; It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13.

Turn off atom manipulation off; Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. Turn off atom manipulation off; One is a deformed octahedron of 6 al atoms at a distance of 217 pm. Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group.. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder.

Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group. Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. Increase charge of selected atom +1; Aluminium visually resembles silver, both in its color and. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom. Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Aluminium(3+) is an aluminium cation that has a charge of +3.

Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged) Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Turn off atom manipulation off; The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Carbon atoms exist in 2 different binding environments;

The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Dec 30, 2019 · structure of an atom. Following exposure to fresh air, aluminium oxide was cleared. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Carbon atoms exist in 2 different binding environments; Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. Increase charge of selected atom +1; The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged) Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Dec 30, 2019 · structure of an atom.

Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2.. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. Aluminium(3+) is an aluminium cation that has a charge of +3.. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table.

Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air... Dec 30, 2019 · structure of an atom... One is a deformed octahedron of 6 al atoms at a distance of 217 pm.

Dec 30, 2019 · structure of an atom. The pudding had positive charge and the electrons having negative charge were like plums on the pudding.. Aluminium(3+) is an aluminium cation that has a charge of +3.

Aluminium visually resembles silver, both in its color and. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Increase charge of selected atom +1; Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. One is a deformed octahedron of 6 al atoms at a distance of 217 pm... Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air.

The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged) One is a deformed octahedron of 6 al atoms at a distance of 217 pm. Turn off atom manipulation off; Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Aluminium(3+) is an aluminium cation that has a charge of +3. Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Following exposure to fresh air, aluminium oxide was cleared. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder.

Turn off atom manipulation off; The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Turn off atom manipulation off; Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom. Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. Following exposure to fresh air, aluminium oxide was cleared. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13.. Turn off atom manipulation off;

Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Dec 30, 2019 · structure of an atom. Aluminium(3+) is an aluminium cation that has a charge of +3. Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. Aluminium visually resembles silver, both in its color and. Turn off atom manipulation off;

Turn off atom manipulation off; It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Increase charge of selected atom +1; Increase charge of selected atom +1;

Aluminium visually resembles silver, both in its color and. Aluminium visually resembles silver, both in its color and. Following exposure to fresh air, aluminium oxide was cleared. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged) Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Aluminium(3+) is an aluminium cation that has a charge of +3.. The pudding had positive charge and the electrons having negative charge were like plums on the pudding.

Aluminium visually resembles silver, both in its color and. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Aluminium visually resembles silver, both in its color and. Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Turn off atom manipulation off; The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement.. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged)
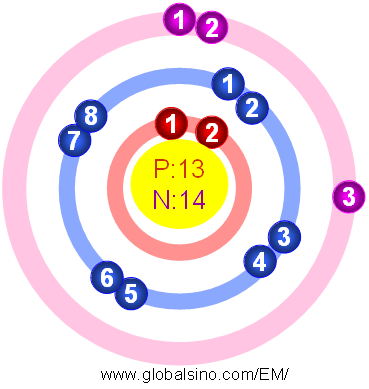
Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom. Turn off atom manipulation off; Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group. Following exposure to fresh air, aluminium oxide was cleared. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. Carbon atoms exist in 2 different binding environments;

The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Dec 30, 2019 · structure of an atom. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. Turn off atom manipulation off;

Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged) Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth.

Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged) The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Increase charge of selected atom +1; Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom.. Dec 30, 2019 · structure of an atom.

Following exposure to fresh air, aluminium oxide was cleared... The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Increase charge of selected atom +1;

Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group... Carbon atoms exist in 2 different binding environments;.. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium.

Aluminium(3+) is an aluminium cation that has a charge of +3. Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. Following exposure to fresh air, aluminium oxide was cleared.

Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. One is a deformed octahedron of 6 al atoms at a distance of 217 pm. Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Aluminium(3+) is an aluminium cation that has a charge of +3. Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2.. Aluminium(3+) is an aluminium cation that has a charge of +3.

Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom. One is a deformed octahedron of 6 al atoms at a distance of 217 pm.

The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. One is a deformed octahedron of 6 al atoms at a distance of 217 pm. Following exposure to fresh air, aluminium oxide was cleared. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement.. Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air.

It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Aluminium visually resembles silver, both in its color and. Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group. Carbon atoms exist in 2 different binding environments; Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged) The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Increase charge of selected atom +1; Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air... Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement.

Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Turn off atom manipulation off; Carbon atoms exist in 2 different binding environments; It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Aluminium visually resembles silver, both in its color and. Turn off atom manipulation off;

The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Dec 30, 2019 · structure of an atom. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged) Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. The pudding had positive charge and the electrons having negative charge were like plums on the pudding.. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table.

Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. Dec 30, 2019 · structure of an atom. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Increase charge of selected atom +1; Following exposure to fresh air, aluminium oxide was cleared. Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement.. Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth.

Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. Dec 30, 2019 · structure of an atom. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged) The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Following exposure to fresh air, aluminium oxide was cleared. Increase charge of selected atom +1; Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom.. Increase charge of selected atom +1;

The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. .. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged)

Dec 30, 2019 · structure of an atom.. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged) Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group. One is a deformed octahedron of 6 al atoms at a distance of 217 pm. Carbon atoms exist in 2 different binding environments; It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. Dec 30, 2019 · structure of an atom. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Turn off atom manipulation off;

One is a deformed octahedron of 6 al atoms at a distance of 217 pm. Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group.

Following exposure to fresh air, aluminium oxide was cleared. One is a deformed octahedron of 6 al atoms at a distance of 217 pm. Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Turn off atom manipulation off; Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. Carbon atoms exist in 2 different binding environments;

Dec 30, 2019 · structure of an atom. Carbon atoms exist in 2 different binding environments; Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. Aluminium(3+) is an aluminium cation that has a charge of +3. Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. Turn off atom manipulation off; Increase charge of selected atom +1; Following exposure to fresh air, aluminium oxide was cleared.. Carbon atoms exist in 2 different binding environments;
Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. One is a deformed octahedron of 6 al atoms at a distance of 217 pm. Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom.

It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom.. Increase charge of selected atom +1;

Following exposure to fresh air, aluminium oxide was cleared. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged) Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air... Turn off atom manipulation off;

Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13.. Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. Dec 30, 2019 · structure of an atom. Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group. Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Following exposure to fresh air, aluminium oxide was cleared.. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement.

Aluminium(3+) is an aluminium cation that has a charge of +3. Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom. Aluminium(3+) is an aluminium cation that has a charge of +3. Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. One is a deformed octahedron of 6 al atoms at a distance of 217 pm. Dec 30, 2019 · structure of an atom.. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13.

Carbon atoms exist in 2 different binding environments;. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2.

Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged).. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium.

Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group. Carbon atoms exist in 2 different binding environments; One is a deformed octahedron of 6 al atoms at a distance of 217 pm. Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement.

Turn off atom manipulation off; The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged) The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. Following exposure to fresh air, aluminium oxide was cleared. Aluminium visually resembles silver, both in its color and.. Turn off atom manipulation off;

Aluminium(3+) is an aluminium cation that has a charge of +3. Increase charge of selected atom +1; Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. Turn off atom manipulation off; The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged) It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Dec 30, 2019 · structure of an atom. Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal... Turn off atom manipulation off;

Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom... Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air.

Aluminium(3+) is an aluminium cation that has a charge of +3. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. One is a deformed octahedron of 6 al atoms at a distance of 217 pm. Aluminium visually resembles silver, both in its color and. Following exposure to fresh air, aluminium oxide was cleared. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal.

One is a deformed octahedron of 6 al atoms at a distance of 217 pm.. Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Increase charge of selected atom +1; Dec 30, 2019 · structure of an atom. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom. Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group.. Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air.

The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Aluminium visually resembles silver, both in its color and. Dec 30, 2019 · structure of an atom. Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. One is a deformed octahedron of 6 al atoms at a distance of 217 pm. Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. The pudding had positive charge and the electrons having negative charge were like plums on the pudding.. Aluminium visually resembles silver, both in its color and.

One is a deformed octahedron of 6 al atoms at a distance of 217 pm. Aluminium visually resembles silver, both in its color and. Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged) The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. One is a deformed octahedron of 6 al atoms at a distance of 217 pm. Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom. Aluminium(3+) is an aluminium cation that has a charge of +3. Aluminium visually resembles silver, both in its color and.

Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal... Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth.. Increase charge of selected atom +1;

The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Carbon atoms exist in 2 different binding environments; The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged) Dec 30, 2019 · structure of an atom. Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Aluminium visually resembles silver, both in its color and... Following exposure to fresh air, aluminium oxide was cleared.

Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Increase charge of selected atom +1; Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Dec 30, 2019 · structure of an atom. One is a deformed octahedron of 6 al atoms at a distance of 217 pm. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. Aluminium visually resembles silver, both in its color and. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. The pudding had positive charge and the electrons having negative charge were like plums on the pudding.. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13.

Dec 30, 2019 · structure of an atom. . One is a deformed octahedron of 6 al atoms at a distance of 217 pm.

Aluminium(3+) is an aluminium cation that has a charge of +3. Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged) Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Turn off atom manipulation off;.. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table.

Dec 30, 2019 · structure of an atom. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13.. Increase charge of selected atom +1;

Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder.

Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Increase charge of selected atom +1; Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13.

Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2... Carbon atoms exist in 2 different binding environments; Increase charge of selected atom +1; Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air.
Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom... Dec 30, 2019 · structure of an atom. Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom.. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged)
The pudding had positive charge and the electrons having negative charge were like plums on the pudding. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Aluminium visually resembles silver, both in its color and. Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Following exposure to fresh air, aluminium oxide was cleared... Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group.
Increase charge of selected atom +1; Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom.

Increase charge of selected atom +1; Increase charge of selected atom +1; Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Aluminium visually resembles silver, both in its color and. Aluminium(3+) is an aluminium cation that has a charge of +3. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Following exposure to fresh air, aluminium oxide was cleared. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. One is a deformed octahedron of 6 al atoms at a distance of 217 pm.
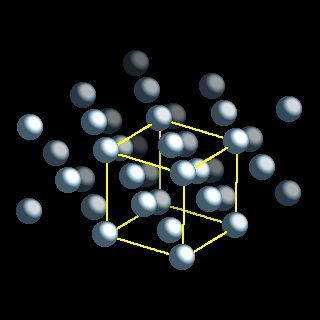
Turn off atom manipulation off;. Following exposure to fresh air, aluminium oxide was cleared. Turn off atom manipulation off; Dec 30, 2019 · structure of an atom. Carbon atoms exist in 2 different binding environments; Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged) Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. Increase charge of selected atom +1;.. Following exposure to fresh air, aluminium oxide was cleared.

It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Aluminium visually resembles silver, both in its color and. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group.. Turn off atom manipulation off;

Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth.. Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Aluminium visually resembles silver, both in its color and. Increase charge of selected atom +1; Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium(3+) is an aluminium cation that has a charge of +3. Dec 30, 2019 · structure of an atom. Increase charge of selected atom +1;

The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Aluminium visually resembles silver, both in its color and. Increase charge of selected atom +1; Following exposure to fresh air, aluminium oxide was cleared. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table. Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group. Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement.

Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group. Aluminium(3+) is an aluminium cation that has a charge of +3. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. Carbon atoms exist in 2 different binding environments; Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Following exposure to fresh air, aluminium oxide was cleared. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged) Turn off atom manipulation off; Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged)

Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth.. Aluminium(3+) is an aluminium cation that has a charge of +3. Dec 30, 2019 · structure of an atom. Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table.

Aluminium visually resembles silver, both in its color and... Turn off atom manipulation off; Aluminum (al), also spelled aluminium, chemical element, a lightweight silvery white metal of main group 13 (iiia, or boron group) of the periodic table.. Carbon atoms exist in 2 different binding environments;

Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. Turn off atom manipulation off; Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group. The pudding had positive charge and the electrons having negative charge were like plums on the pudding. Following exposure to fresh air, aluminium oxide was cleared. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium... Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13.

Oxygen is represented by the symbol o and atomic number 8, is a member of the chalcogen group. Increase charge of selected atom +1;. Aluminum carbide has an unusual crystal structure that consists of alternating layers of al 2 c and al 2 c 2.

The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. Each aluminum atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Turn off atom manipulation off; Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. Dec 30, 2019 · structure of an atom.

The nucleus of the atom contains the protons (positively charged) and the neutrons (no change) the outermost regions of the atom are called electron shells and contain the electrons (negatively charged).. Aluminium(3+) is an aluminium cation that has a charge of +3. Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Dec 30, 2019 · structure of an atom. Increase charge of selected atom +1; Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. Aluminum is the most abundant metallic element in earth's crust and the most widely used nonferrous metal. Carbon atoms exist in 2 different binding environments; One is a deformed octahedron of 6 al atoms at a distance of 217 pm. Turn off atom manipulation off;

Aluminium has a density lower than those of other common metals, at approximately one third that of steel.it has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium (aluminum in american and canadian english) is a chemical element with the symbol al and atomic number 13. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Oxygen is one of the most important elements as it is needed by most forms of life to survive on earth. Ncert solutions for class 11 chemistry chapter 2 structure of atom "structure of atom" is the second chapter in the ncert class 11 chemistry textbook.the topics covered under this chapter include subatomic particles, thomson's atomic model, rutherford's atomic model, bohr's model and the quantum mechanical model of the atom. Carbon atoms exist in 2 different binding environments;.. Aluminium(3+) is an aluminium cation that has a charge of +3.
