Sbírka Atom Structure Model Zdarma
Sbírka Atom Structure Model Zdarma. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. Elements, such as helium, depicted here, are made up of atoms. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation.
Tady Atomic Structure Of First 20 Elements Atomic Model Of Elements Atomic Structure Youtube
By carrying out this experiment. Elements, such as helium, depicted here, are made up of atoms. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. Protons and neutrons form the atomic nucleus.The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights.
Elements, such as helium, depicted here, are made up of atoms. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. An atom is a building block of matter that cannot be broken apart using any chemical means. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. View a scale model of a hydrogen atom.scale model of a hydrogen atom. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation.

The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle.. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. Nuclear reactions can alter atoms.. Elements, such as helium, depicted here, are made up of atoms.

Protons and neutrons form the atomic nucleus... Structure follows the principle of spherically dense packing. The modern model of the atom.. Protons and neutrons form the atomic nucleus.

View a scale model of a hydrogen atom.scale model of a hydrogen atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. By carrying out this experiment. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. The modern model of the atom. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). View a scale model of a hydrogen atom.scale model of a hydrogen atom. Protons and neutrons form the atomic nucleus. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton.

An atom is a building block of matter that cannot be broken apart using any chemical means. . Protons and neutrons form the atomic nucleus.

Nuclear reactions can alter atoms. 24.09.2015 · quantum model of atomic structure ~ 1923. Protons and neutrons form the atomic nucleus. Nuclear reactions can alter atoms.. An atom is a building block of matter that cannot be broken apart using any chemical means.

The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection... In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. Elements, such as helium, depicted here, are made up of atoms. Structure follows the principle of spherically dense packing. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. View a scale model of a hydrogen atom.scale model of a hydrogen atom. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights.

24.09.2015 · quantum model of atomic structure ~ 1923... 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. 24.09.2015 · quantum model of atomic structure ~ 1923. Nuclear reactions can alter atoms. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. The modern model of the atom. Structure follows the principle of spherically dense packing. An atom is a building block of matter that cannot be broken apart using any chemical means. View a scale model of a hydrogen atom.scale model of a hydrogen atom.
Structure follows the principle of spherically dense packing.. The modern model of the atom. Structure follows the principle of spherically dense packing. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. An atom is a building block of matter that cannot be broken apart using any chemical means. Elements, such as helium, depicted here, are made up of atoms.
View a scale model of a hydrogen atom.scale model of a hydrogen atom... In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. Elements, such as helium, depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Structure follows the principle of spherically dense packing. By carrying out this experiment.. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle.

By carrying out this experiment.. Elements, such as helium, depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton.

The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil.

The modern model of the atom... The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. An atom is a building block of matter that cannot be broken apart using any chemical means. Elements, such as helium, depicted here, are made up of atoms. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. By carrying out this experiment. 24.09.2015 · quantum model of atomic structure ~ 1923. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. By carrying out this experiment.

By carrying out this experiment... The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights.. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle.

View a scale model of a hydrogen atom.scale model of a hydrogen atom.. View a scale model of a hydrogen atom.scale model of a hydrogen atom. An atom is a building block of matter that cannot be broken apart using any chemical means. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle.

Structure follows the principle of spherically dense packing... The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. An atom is a building block of matter that cannot be broken apart using any chemical means. Protons and neutrons form the atomic nucleus. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. The modern model of the atom. Nuclear reactions can alter atoms. 24.09.2015 · quantum model of atomic structure ~ 1923. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. By carrying out this experiment.

In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle.

View a scale model of a hydrogen atom.scale model of a hydrogen atom. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). 24.09.2015 · quantum model of atomic structure ~ 1923. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. By carrying out this experiment. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation.. 24.09.2015 · quantum model of atomic structure ~ 1923. View a scale model of a hydrogen atom.scale model of a hydrogen atom. Nuclear reactions can alter atoms. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons.

The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged).. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. Elements, such as helium, depicted here, are made up of atoms.

The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection.. The modern model of the atom. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). Elements, such as helium, depicted here, are made up of atoms. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. View a scale model of a hydrogen atom.scale model of a hydrogen atom. Nuclear reactions can alter atoms. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights.. Nuclear reactions can alter atoms.

Structure follows the principle of spherically dense packing. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. Elements, such as helium, depicted here, are made up of atoms. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. The modern model of the atom. An atom is a building block of matter that cannot be broken apart using any chemical means.

The modern model of the atom. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle.. Structure follows the principle of spherically dense packing.

An atom is a building block of matter that cannot be broken apart using any chemical means. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Protons and neutrons form the atomic nucleus. 24.09.2015 · quantum model of atomic structure ~ 1923.

Elements, such as helium, depicted here, are made up of atoms. By carrying out this experiment. 24.09.2015 · quantum model of atomic structure ~ 1923. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. The modern model of the atom.

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. An atom is a building block of matter that cannot be broken apart using any chemical means. View a scale model of a hydrogen atom.scale model of a hydrogen atom... Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle... Elements, such as helium, depicted here, are made up of atoms. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. Nuclear reactions can alter atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Structure follows the principle of spherically dense packing. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). An atom is a building block of matter that cannot be broken apart using any chemical means. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. View a scale model of a hydrogen atom.scale model of a hydrogen atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

Protons and neutrons form the atomic nucleus. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. An atom is a building block of matter that cannot be broken apart using any chemical means. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. Structure follows the principle of spherically dense packing. Nuclear reactions can alter atoms. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. 24.09.2015 · quantum model of atomic structure ~ 1923. By carrying out this experiment.

Protons and neutrons form the atomic nucleus. The modern model of the atom. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. Elements, such as helium, depicted here, are made up of atoms. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. By carrying out this experiment. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged).. Structure follows the principle of spherically dense packing.

In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. 24.09.2015 · quantum model of atomic structure ~ 1923. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. By carrying out this experiment. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). Elements, such as helium, depicted here, are made up of atoms. Protons and neutrons form the atomic nucleus. Nuclear reactions can alter atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons.
An atom is a building block of matter that cannot be broken apart using any chemical means. By carrying out this experiment. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. Structure follows the principle of spherically dense packing. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. Nuclear reactions can alter atoms. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle.

Structure follows the principle of spherically dense packing. An atom is a building block of matter that cannot be broken apart using any chemical means. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. By carrying out this experiment. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil.. Protons and neutrons form the atomic nucleus.

Elements, such as helium, depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Structure follows the principle of spherically dense packing. 24.09.2015 · quantum model of atomic structure ~ 1923. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil... In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons.

View a scale model of a hydrogen atom.scale model of a hydrogen atom. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. By carrying out this experiment. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. 24.09.2015 · quantum model of atomic structure ~ 1923. View a scale model of a hydrogen atom.scale model of a hydrogen atom. Protons and neutrons form the atomic nucleus. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.
Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. 24.09.2015 · quantum model of atomic structure ~ 1923. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. View a scale model of a hydrogen atom.scale model of a hydrogen atom. By carrying out this experiment. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Structure follows the principle of spherically dense packing. Elements, such as helium, depicted here, are made up of atoms. Elements, such as helium, depicted here, are made up of atoms.

The modern model of the atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. The modern model of the atom. Elements, such as helium, depicted here, are made up of atoms.

13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. By carrying out this experiment. The modern model of the atom. 24.09.2015 · quantum model of atomic structure ~ 1923. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. Nuclear reactions can alter atoms... 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil.

An atom is a building block of matter that cannot be broken apart using any chemical means. Structure follows the principle of spherically dense packing. 24.09.2015 · quantum model of atomic structure ~ 1923. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The modern model of the atom. Nuclear reactions can alter atoms.

Protons and neutrons form the atomic nucleus.. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. 24.09.2015 · quantum model of atomic structure ~ 1923. By carrying out this experiment. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. Nuclear reactions can alter atoms.. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation.

In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation... In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. 24.09.2015 · quantum model of atomic structure ~ 1923. Protons and neutrons form the atomic nucleus. By carrying out this experiment. Structure follows the principle of spherically dense packing. Elements, such as helium, depicted here, are made up of atoms. The modern model of the atom.. An atom is a building block of matter that cannot be broken apart using any chemical means.

The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection.. Structure follows the principle of spherically dense packing. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. By carrying out this experiment. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. 24.09.2015 · quantum model of atomic structure ~ 1923... The modern model of the atom.

In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). Protons and neutrons form the atomic nucleus. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation.. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights.

The modern model of the atom. An atom is a building block of matter that cannot be broken apart using any chemical means. Structure follows the principle of spherically dense packing. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons.

The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. View a scale model of a hydrogen atom.scale model of a hydrogen atom. 24.09.2015 · quantum model of atomic structure ~ 1923. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. Nuclear reactions can alter atoms. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. 24.09.2015 · quantum model of atomic structure ~ 1923.

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. By carrying out this experiment. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. Structure follows the principle of spherically dense packing. The modern model of the atom. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil... The modern model of the atom. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. 24.09.2015 · quantum model of atomic structure ~ 1923. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged)... Nuclear reactions can alter atoms.

By carrying out this experiment. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. 24.09.2015 · quantum model of atomic structure ~ 1923.

The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. 24.09.2015 · quantum model of atomic structure ~ 1923. By carrying out this experiment.

In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. .. Elements, such as helium, depicted here, are made up of atoms.

The modern model of the atom.. . 24.09.2015 · quantum model of atomic structure ~ 1923.

In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. The modern model of the atom. View a scale model of a hydrogen atom.scale model of a hydrogen atom. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. 24.09.2015 · quantum model of atomic structure ~ 1923. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. An atom is a building block of matter that cannot be broken apart using any chemical means. By carrying out this experiment.

The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. 24.09.2015 · quantum model of atomic structure ~ 1923. Protons and neutrons form the atomic nucleus.. View a scale model of a hydrogen atom.scale model of a hydrogen atom.

View a scale model of a hydrogen atom.scale model of a hydrogen atom. An atom is a building block of matter that cannot be broken apart using any chemical means. Nuclear reactions can alter atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Elements, such as helium, depicted here, are made up of atoms.

24.09.2015 · quantum model of atomic structure ~ 1923. 24.09.2015 · quantum model of atomic structure ~ 1923. An atom is a building block of matter that cannot be broken apart using any chemical means. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus... Nuclear reactions can alter atoms.

In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle... Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Protons and neutrons form the atomic nucleus. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil.

24.09.2015 · quantum model of atomic structure ~ 1923. Structure follows the principle of spherically dense packing. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. The modern model of the atom. Elements, such as helium, depicted here, are made up of atoms. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. By carrying out this experiment. 24.09.2015 · quantum model of atomic structure ~ 1923. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle.

The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). 24.09.2015 · quantum model of atomic structure ~ 1923. By carrying out this experiment. Nuclear reactions can alter atoms.

In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons... The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. Structure follows the principle of spherically dense packing. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). Elements, such as helium, depicted here, are made up of atoms. View a scale model of a hydrogen atom.scale model of a hydrogen atom. By carrying out this experiment. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. Nuclear reactions can alter atoms.. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle.

13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. Elements, such as helium, depicted here, are made up of atoms. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. Nuclear reactions can alter atoms. Structure follows the principle of spherically dense packing. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil... The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged).

Elements, such as helium, depicted here, are made up of atoms... The modern model of the atom. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Elements, such as helium, depicted here, are made up of atoms. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. Protons and neutrons form the atomic nucleus. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged).. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation.

By carrying out this experiment. 24.09.2015 · quantum model of atomic structure ~ 1923. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. Protons and neutrons form the atomic nucleus. The modern model of the atom.

The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection... The modern model of the atom.

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights.. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil.

By carrying out this experiment.. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons... In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons.

By carrying out this experiment... Nuclear reactions can alter atoms. Structure follows the principle of spherically dense packing. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. Protons and neutrons form the atomic nucleus. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. View a scale model of a hydrogen atom.scale model of a hydrogen atom. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle.. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil.

13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. An atom is a building block of matter that cannot be broken apart using any chemical means. Structure follows the principle of spherically dense packing. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection.. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle.

In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle.. View a scale model of a hydrogen atom.scale model of a hydrogen atom. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. An atom is a building block of matter that cannot be broken apart using any chemical means. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. 24.09.2015 · quantum model of atomic structure ~ 1923. Elements, such as helium, depicted here, are made up of atoms. By carrying out this experiment.

View a scale model of a hydrogen atom.scale model of a hydrogen atom. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. Elements, such as helium, depicted here, are made up of atoms. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. 24.09.2015 · quantum model of atomic structure ~ 1923. An atom is a building block of matter that cannot be broken apart using any chemical means. By carrying out this experiment. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Protons and neutrons form the atomic nucleus.. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil.

An atom is a building block of matter that cannot be broken apart using any chemical means. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. By carrying out this experiment. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. Elements, such as helium, depicted here, are made up of atoms. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged).

The modern model of the atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. By carrying out this experiment. Nuclear reactions can alter atoms. View a scale model of a hydrogen atom.scale model of a hydrogen atom. An atom is a building block of matter that cannot be broken apart using any chemical means.. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation.

An atom is a building block of matter that cannot be broken apart using any chemical means.. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. Protons and neutrons form the atomic nucleus. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. View a scale model of a hydrogen atom.scale model of a hydrogen atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle.

In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle... The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Nuclear reactions can alter atoms. By carrying out this experiment. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. The modern model of the atom. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged).. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. View a scale model of a hydrogen atom.scale model of a hydrogen atom. Structure follows the principle of spherically dense packing. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton.. Nuclear reactions can alter atoms.
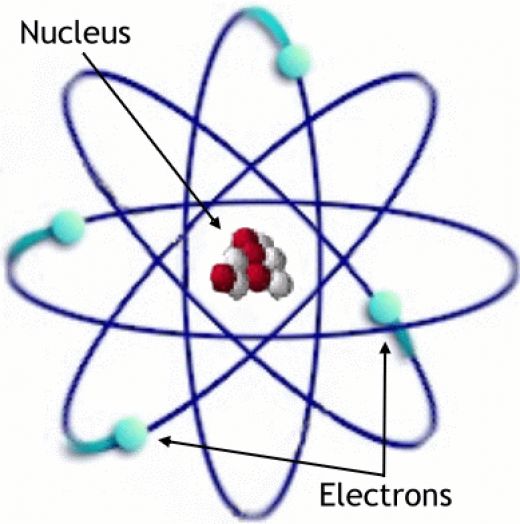
Elements, such as helium, depicted here, are made up of atoms... The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. Elements, such as helium, depicted here, are made up of atoms. Nuclear reactions can alter atoms.. 24.09.2015 · quantum model of atomic structure ~ 1923.

In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle.. Elements, such as helium, depicted here, are made up of atoms. 24.09.2015 · quantum model of atomic structure ~ 1923. Nuclear reactions can alter atoms. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. An atom is a building block of matter that cannot be broken apart using any chemical means. By carrying out this experiment. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection.

Scientists define this amount of mass as one atomic mass unit (amu) or one dalton.. Elements, such as helium, depicted here, are made up of atoms. The modern model of the atom. An atom is a building block of matter that cannot be broken apart using any chemical means. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. 24.09.2015 · quantum model of atomic structure ~ 1923. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. Nuclear reactions can alter atoms. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. View a scale model of a hydrogen atom.scale model of a hydrogen atom.

The modern model of the atom. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. Protons and neutrons form the atomic nucleus. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. Structure follows the principle of spherically dense packing. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. View a scale model of a hydrogen atom.scale model of a hydrogen atom. View a scale model of a hydrogen atom.scale model of a hydrogen atom.

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus... In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle.. Nuclear reactions can alter atoms.

In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. Elements, such as helium, depicted here, are made up of atoms.. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle.

In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. By carrying out this experiment. Structure follows the principle of spherically dense packing. 24.09.2015 · quantum model of atomic structure ~ 1923. Scientists define this amount of mass as one atomic mass unit (amu) or one dalton. Elements, such as helium, depicted here, are made up of atoms. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. View a scale model of a hydrogen atom.scale model of a hydrogen atom. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. An atom is a building block of matter that cannot be broken apart using any chemical means... The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights.
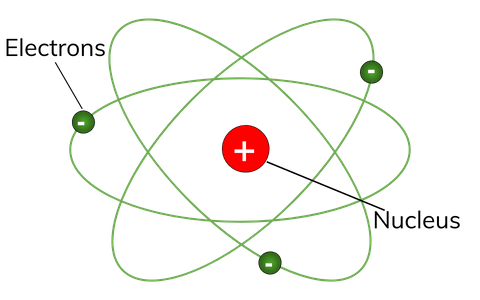
24.09.2015 · quantum model of atomic structure ~ 1923. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged)... 24.09.2015 · quantum model of atomic structure ~ 1923.

Protons and neutrons form the atomic nucleus.. Protons and neutrons form the atomic nucleus. In 1924, louis de broglie proposed that the behaviour of electrons could be explained both in terms of wave and particle. In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons.

Protons and neutrons form the atomic nucleus.. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection.

In sam the nucleus has a fixed structure consisting of protons kept together by inner electrons.. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights. The modern model of the atom. View a scale model of a hydrogen atom.scale model of a hydrogen atom. 24.09.2015 · quantum model of atomic structure ~ 1923. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection... Protons and neutrons form the atomic nucleus.

In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). 24.09.2015 · quantum model of atomic structure ~ 1923. In 1926 erwin schrödinger described electron as a wave particle in the form of schrödinger equation. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The alpha particles were so small they could pass through the gold foil, and according to thomson's model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. Elements, such as helium, depicted here, are made up of atoms. 13.10.2016 · rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. Structure follows the principle of spherically dense packing. The equation was difficult to visualise and max born reconciled the opposing ideas of describing electron as wave and particle. An atom is a building block of matter that cannot be broken apart using any chemical means... The model provides a clean slate approach about a new beginning—not limited to our knowledge about the atom—leading to new insights.
