Atom Vs Molecule
Atom Vs Molecule. Molecule, you know the atom came first. While there are other fun subatomic particle names thrown in there, those are the three. Atom may or may not exist in the free state while molecule always exists in the free state.
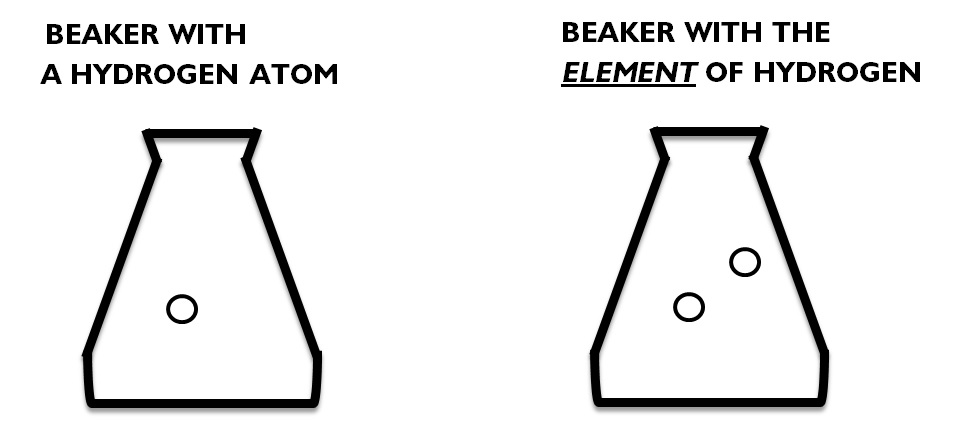
Prezentováno Basic Difference Between An Atom And A Molecule
On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state. An atom is the smallest unit of matter. 14.05.2017 · this chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. It also explains how to distinguish ionic and molecular.Two or more atoms held together by.
Atom may or may not exist in the free state while molecule always exists in the free state. In chemistry, there are two main types of particle that we need to know about. 25.02.2021 · 12 key differences (atoms vs molecules) characteristics: On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state. The atom comprises of the … To know the answer to that question, you need to look at the structure of an atom.

Contrary to this, the molecule is less reactive and more stable. The atom is the smallest particle of an element that may or may not exists independently. Now the only question is why? On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state. So, what is the difference … Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound; Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound;
Atoms may or may not exist in the free state, but molecules exist in the free state. 14.05.2017 · this chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. Atoms may or may not exist in the free state, but molecules exist in the free state. Now the only question is why? The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons. Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound; Contrary to this, the molecule is less reactive and more stable. The atom is reactive as it is less stable as compared to the molecule.. But when it comes to an atom vs.

Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound; In chemistry, there are different terms to describe the structure of matter, such as atom vs. Two or more atoms held together by. So, what is the difference … Now the only question is why? 07.12.2018 · the difference between atom and molecule can be drawn clearly on the following grounds: In chemistry, there are two main types of particle that we need to know about.. On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state.
Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound;.. 25.02.2021 · 12 key differences (atoms vs molecules) characteristics: Contrary to this, the molecule is less reactive and more stable. It's composed of different particles called electrons, protons, and neutrons. Atom is defined as the smallest unit of an element which may or may not exists independently. Atom may or may not exist in the free state while molecule always exists in the free state.. Contrary to this, the molecule is less reactive and more stable.

Molecule, you know the atom came first.. Two or more atoms held together by. Atom may or may not exist in the free state while molecule always exists in the free state. While there are other fun subatomic particle names thrown in there, those are the three. 14.05.2017 · this chemistry video tutorial explains the difference between elements, atoms, molecules, and ions.

But when it comes to an atom vs. Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound; Molecule, you know the atom came first. To know the answer to that question, you need to look at the structure of an atom. 14.05.2017 · this chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state. All matter in the universe is made up of different particles. In chemistry, there are different terms to describe the structure of matter, such as atom vs. The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons. An atom is the smallest unit of matter composed of a nucleus and one or more electrons present around the nucleus. Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound;

14.05.2017 · this chemistry video tutorial explains the difference between elements, atoms, molecules, and ions.. Molecule, you know the atom came first. 07.12.2018 · the difference between atom and molecule can be drawn clearly on the following grounds: Contrary to this, the molecule comprises two or more than two atoms. So, what is the difference … Atom may or may not exist in the free state while molecule always exists in the free state.. While there are other fun subatomic particle names thrown in there, those are the three.

It also explains how to distinguish ionic and molecular.. So, what is the difference …

An atom is the smallest unit of matter. On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state. It's composed of different particles called electrons, protons, and neutrons. Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound; To know the answer to that question, you need to look at the structure of an atom. 07.12.2018 · the difference between atom and molecule can be drawn clearly on the following grounds: The atom is the smallest particle of an element that may or may not exists independently. A molecule is a group of two or more atoms that represent the smallest identifiable unit of a pure substance and retains the composition and chemical properties of the substance. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound.. Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound;

On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state. The atom is reactive as it is less stable as compared to the molecule. Atoms may or may not exist in the free state, but molecules exist in the free state. 07.12.2018 · the difference between atom and molecule can be drawn clearly on the following grounds: The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons. Contrary to this, the molecule comprises two or more than two atoms. While there are other fun subatomic particle names thrown in there, those are the three. The atom is the smallest particle of an element that may or may not exists independently. Atom is defined as the smallest unit of an element which may or may not exists independently. Atom may or may not exist in the free state while molecule always exists in the free state. It also explains how to distinguish ionic and molecular... Atom is defined as the smallest unit of an element which may or may not exists independently.

In chemistry, there are two main types of particle that we need to know about. To know the answer to that question, you need to look at the structure of an atom. 14.05.2017 · this chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. In chemistry, there are different terms to describe the structure of matter, such as atom vs. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state. In chemistry, there are two main types of particle that we need to know about. Atom may or may not exist in the free state while molecule always exists in the free state.. 07.12.2018 · the difference between atom and molecule can be drawn clearly on the following grounds:

The atom is the smallest particle of an element that may or may not exists independently. All matter in the universe is made up of different particles. A molecule is a group of two or more atoms that represent the smallest identifiable unit of a pure substance and retains the composition and chemical properties of the substance. On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state. Now the only question is why? Atom is defined as the smallest unit of an element which may or may not exists independently. It's composed of different particles called electrons, protons, and neutrons.. It's composed of different particles called electrons, protons, and neutrons.

In chemistry, there are different terms to describe the structure of matter, such as atom vs. All matter in the universe is made up of different particles. Atom is defined as the smallest unit of an element which may or may not exists independently.

In chemistry, there are two main types of particle that we need to know about.. Contrary to this, the molecule comprises two or more than two atoms. All matter in the universe is made up of different particles. 07.12.2018 · the difference between atom and molecule can be drawn clearly on the following grounds: The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons. Now the only question is why? It's composed of different particles called electrons, protons, and neutrons. Atom may or may not exist in the free state while molecule always exists in the free state. 14.05.2017 · this chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. Atom is defined as the smallest unit of an element which may or may not exists independently. An atom is the smallest unit of matter composed of a nucleus and one or more electrons present around the nucleus.. Atom is defined as the smallest unit of an element which may or may not exists independently.

Now the only question is why? Atom is the smallest unit of the chemical element, whereas two or more atoms combine to make up the molecule. So, what is the difference …

So, what is the difference … . All matter in the universe is made up of different particles.
The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons. Molecule, you know the atom came first. It also explains how to distinguish ionic and molecular. Now the only question is why? All matter in the universe is made up of different particles. On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state. So, what is the difference … To know the answer to that question, you need to look at the structure of an atom. In chemistry, there are different terms to describe the structure of matter, such as atom vs.. In chemistry, there are two main types of particle that we need to know about.

Molecule, you know the atom came first. The atom comprises of the … The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons. Molecule, you know the atom came first.

To know the answer to that question, you need to look at the structure of an atom. On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state. Atom is the smallest unit of the chemical element, whereas two or more atoms combine to make up the molecule. Atom may or may not exist in the free state while molecule always exists in the free state. 07.12.2018 · the difference between atom and molecule can be drawn clearly on the following grounds:. So, what is the difference …

Atoms may or may not exist in the free state, but molecules exist in the free state. While there are other fun subatomic particle names thrown in there, those are the three. In chemistry, there are two main types of particle that we need to know about.. Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound;

Contrary to this, the molecule is less reactive and more stable. The atom is reactive as it is less stable as compared to the molecule. Two or more atoms held together by. Now the only question is why? So, what is the difference … Atoms may or may not exist in the free state, but molecules exist in the free state. To know the answer to that question, you need to look at the structure of an atom. Molecule, you know the atom came first.

14.05.2017 · this chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. 07.12.2018 · the difference between atom and molecule can be drawn clearly on the following grounds: Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound; It also explains how to distinguish ionic and molecular. In chemistry, there are two main types of particle that we need to know about.

On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state... Molecule, you know the atom came first. To know the answer to that question, you need to look at the structure of an atom. Atoms may or may not exist in the free state, but molecules exist in the free state. The atom comprises of the … In chemistry, there are two main types of particle that we need to know about. Now the only question is why? Contrary to this, the molecule comprises two or more than two atoms... But when it comes to an atom vs.

Atom is the smallest unit of the chemical element, whereas two or more atoms combine to make up the molecule. An atom is the smallest unit of matter. 25.02.2021 · 12 key differences (atoms vs molecules) characteristics: Now the only question is why? It also explains how to distinguish ionic and molecular. 14.05.2017 · this chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. An atom is the smallest unit of matter composed of a nucleus and one or more electrons present around the nucleus. But when it comes to an atom vs.

In chemistry, there are two main types of particle that we need to know about. 25.02.2021 · 12 key differences (atoms vs molecules) characteristics: The atom comprises of the … An atom is the smallest unit of matter composed of a nucleus and one or more electrons present around the nucleus. While there are other fun subatomic particle names thrown in there, those are the three. On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state. Atom may or may not exist in the free state while molecule always exists in the free state. The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons. The atom is the smallest particle of an element that may or may not exists independently. Atoms may or may not exist in the free state, but molecules exist in the free state. All matter in the universe is made up of different particles. The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons.

The atom is the smallest particle of an element that may or may not exists independently. But when it comes to an atom vs. Atoms may or may not exist in the free state, but molecules exist in the free state. Two or more atoms held together by. Molecule, you know the atom came first. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. So, what is the difference … An atom is the smallest unit of matter. Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound; The atom is the smallest particle of an element that may or may not exists independently. The atom comprises of the … An atom is the smallest unit of matter.

To know the answer to that question, you need to look at the structure of an atom. In chemistry, there are two main types of particle that we need to know about. Contrary to this, the molecule is less reactive and more stable. An atom is the smallest unit of matter. But when it comes to an atom vs. Atom is the smallest unit of the chemical element, whereas two or more atoms combine to make up the molecule. To know the answer to that question, you need to look at the structure of an atom. It also explains how to distinguish ionic and molecular. An atom is the smallest unit of matter composed of a nucleus and one or more electrons present around the nucleus. Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound;

To know the answer to that question, you need to look at the structure of an atom. 07.12.2018 · the difference between atom and molecule can be drawn clearly on the following grounds: The atom is the smallest particle of an element that may or may not exists independently. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. Atom is defined as the smallest unit of an element which may or may not exists independently. Two or more atoms held together by. While there are other fun subatomic particle names thrown in there, those are the three. Atom may or may not exist in the free state while molecule always exists in the free state.. So, what is the difference …

It also explains how to distinguish ionic and molecular... But when it comes to an atom vs. Two or more atoms held together by. The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons.

Contrary to this, the molecule is less reactive and more stable. The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons. In chemistry, there are different terms to describe the structure of matter, such as atom vs.

Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound; On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. It also explains how to distinguish ionic and molecular. 14.05.2017 · this chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. The atom is the smallest particle of an element that may or may not exists independently. But when it comes to an atom vs.. While there are other fun subatomic particle names thrown in there, those are the three.

A molecule is a group of two or more atoms that represent the smallest identifiable unit of a pure substance and retains the composition and chemical properties of the substance. Molecule, you know the atom came first. All matter in the universe is made up of different particles. Contrary to this, the molecule is less reactive and more stable. On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state. 14.05.2017 · this chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. Now the only question is why? The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons. 25.02.2021 · 12 key differences (atoms vs molecules) characteristics: An atom is the smallest unit of matter composed of a nucleus and one or more electrons present around the nucleus.

Now the only question is why?. But when it comes to an atom vs. The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons. 14.05.2017 · this chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. Atom is the smallest unit of the chemical element, whereas two or more atoms combine to make up the molecule. The atom is the smallest particle of an element that may or may not exists independently. Atom is defined as the smallest unit of an element which may or may not exists independently. An atom is the smallest unit of matter. 07.12.2018 · the difference between atom and molecule can be drawn clearly on the following grounds: 25.02.2021 · 12 key differences (atoms vs molecules) characteristics: It's composed of different particles called electrons, protons, and neutrons. Atom is defined as the smallest unit of an element which may or may not exists independently.

25.02.2021 · 12 key differences (atoms vs molecules) characteristics: Atom is defined as the smallest unit of an element which may or may not exists independently. An atom is the smallest unit of matter. Atom is the smallest unit of the chemical element, whereas two or more atoms combine to make up the molecule. So, what is the difference … Atom is defined as the smallest unit of an element which may or may not exists independently.

Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound;. All matter in the universe is made up of different particles. While there are other fun subatomic particle names thrown in there, those are the three. Two or more atoms held together by. An atom is the smallest unit of matter composed of a nucleus and one or more electrons present around the nucleus. 25.02.2021 · 12 key differences (atoms vs molecules) characteristics: On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. The atom is reactive as it is less stable as compared to the molecule.

In chemistry, there are different terms to describe the structure of matter, such as atom vs. Atoms may or may not exist in the free state, but molecules exist in the free state. The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons... An atom is the smallest unit of matter.

The atom is the smallest particle of an element that may or may not exists independently. The atom is reactive as it is less stable as compared to the molecule. Atoms may or may not exist in the free state, but molecules exist in the free state. 25.02.2021 · 12 key differences (atoms vs molecules) characteristics: All matter in the universe is made up of different particles. While there are other fun subatomic particle names thrown in there, those are the three. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. In chemistry, there are different terms to describe the structure of matter, such as atom vs. An atom is the smallest unit of matter. Contrary to this, the molecule comprises two or more than two atoms. But when it comes to an atom vs.

The atom comprises of the …. All matter in the universe is made up of different particles. So, what is the difference … To know the answer to that question, you need to look at the structure of an atom. Atoms may or may not exist in the free state, but molecules exist in the free state. In chemistry, there are two main types of particle that we need to know about. The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons. It also explains how to distinguish ionic and molecular. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. In chemistry, there are different terms to describe the structure of matter, such as atom vs.

The atom is reactive as it is less stable as compared to the molecule. Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound; A molecule is a group of two or more atoms that represent the smallest identifiable unit of a pure substance and retains the composition and chemical properties of the substance. Molecule, you know the atom came first. On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. Atom is the smallest unit of the chemical element, whereas two or more atoms combine to make up the molecule. Atoms may or may not exist in the free state, but molecules exist in the free state. In chemistry, there are two main types of particle that we need to know about... Atom is defined as the smallest unit of an element which may or may not exists independently.

07.12.2018 · the difference between atom and molecule can be drawn clearly on the following grounds:. Atom may or may not exist in the free state while molecule always exists in the free state. While there are other fun subatomic particle names thrown in there, those are the three. An atom is the smallest unit of matter. All matter in the universe is made up of different particles.. The atom is reactive as it is less stable as compared to the molecule.

The atom comprises of the …. To know the answer to that question, you need to look at the structure of an atom. 07.12.2018 · the difference between atom and molecule can be drawn clearly on the following grounds: It also explains how to distinguish ionic and molecular. On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state. Now the only question is why?. But when it comes to an atom vs.

Contrary to this, the molecule comprises two or more than two atoms. Contrary to this, the molecule is less reactive and more stable. The atom is reactive as it is less stable as compared to the molecule. But when it comes to an atom vs. The atom comprises of the … In chemistry, there are different terms to describe the structure of matter, such as atom vs. All matter in the universe is made up of different particles. So, what is the difference …

All matter in the universe is made up of different particles. Contrary to this, the molecule comprises two or more than two atoms. The atom is the smallest particle of an element that may or may not exists independently. An atom is the smallest unit of matter composed of a nucleus and one or more electrons present around the nucleus. Atom may or may not exist in the free state while molecule always exists in the free state. Atom is the smallest unit of the chemical element, whereas two or more atoms combine to make up the molecule. Molecule, you know the atom came first. Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound; On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. In chemistry, there are two main types of particle that we need to know about. The atom is reactive as it is less stable as compared to the molecule.. An atom is the smallest unit of matter.

The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons. Contrary to this, the molecule is less reactive and more stable. Atom is the smallest unit of the chemical element, whereas two or more atoms combine to make up the molecule. So, what is the difference … An atom is the smallest unit of matter composed of a nucleus and one or more electrons present around the nucleus. The atom comprises of the … Two or more atoms held together by. 07.12.2018 · the difference between atom and molecule can be drawn clearly on the following grounds:. It also explains how to distinguish ionic and molecular.

25.02.2021 · 12 key differences (atoms vs molecules) characteristics:.. Atom may or may not exist in the free state while molecule always exists in the free state. Contrary to this, the molecule is less reactive and more stable. While there are other fun subatomic particle names thrown in there, those are the three. The atom comprises of the … All matter in the universe is made up of different particles. 07.12.2018 · the difference between atom and molecule can be drawn clearly on the following grounds: Contrary to this, the molecule comprises two or more than two atoms. Atom is the smallest unit of the chemical element, whereas two or more atoms combine to make up the molecule. The atom is reactive as it is less stable as compared to the molecule.. An atom is the smallest unit of matter composed of a nucleus and one or more electrons present around the nucleus.

Now the only question is why? On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state.. Atoms may or may not exist in the free state, but molecules exist in the free state.

Atom may or may not exist in the free state while molecule always exists in the free state.. In chemistry, there are different terms to describe the structure of matter, such as atom vs. Two or more atoms held together by. An atom is the smallest unit of matter. Contrary to this, the molecule is less reactive and more stable. An atom is the smallest unit of matter composed of a nucleus and one or more electrons present around the nucleus. Molecule, you know the atom came first.. Atoms may or may not exist in the free state, but molecules exist in the free state.

To know the answer to that question, you need to look at the structure of an atom. .. In chemistry, there are different terms to describe the structure of matter, such as atom vs.

The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons. Now the only question is why? 14.05.2017 · this chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. The atom comprises of the … Molecule, you know the atom came first. 25.02.2021 · 12 key differences (atoms vs molecules) characteristics:. 07.12.2018 · the difference between atom and molecule can be drawn clearly on the following grounds:

The atom is reactive as it is less stable as compared to the molecule. But when it comes to an atom vs. On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state. All matter in the universe is made up of different particles. Atom is the smallest unit of the chemical element, whereas two or more atoms combine to make up the molecule. The atom is reactive as it is less stable as compared to the molecule. Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound; Two or more atoms held together by. Contrary to this, the molecule comprises two or more than two atoms. An atom is the smallest unit of matter. To know the answer to that question, you need to look at the structure of an atom. To know the answer to that question, you need to look at the structure of an atom.

Now the only question is why?. The atom is the smallest particle of an element that may or may not exists independently. Two or more atoms held together by. An atom is the smallest unit of matter. The atom comprises of the … The atom is reactive as it is less stable as compared to the molecule. All matter in the universe is made up of different particles. 14.05.2017 · this chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state.. The atom is reactive as it is less stable as compared to the molecule.

A molecule is a group of two or more atoms that represent the smallest identifiable unit of a pure substance and retains the composition and chemical properties of the substance. An atom is the smallest unit of matter composed of a nucleus and one or more electrons present around the nucleus. The atom is the smallest particle of an element that may or may not exists independently. Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound; 07.12.2018 · the difference between atom and molecule can be drawn clearly on the following grounds: Molecule, you know the atom came first. It's composed of different particles called electrons, protons, and neutrons. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. A molecule is a group of two or more atoms that represent the smallest identifiable unit of a pure substance and retains the composition and chemical properties of the substance. The atom comprises of the …. To know the answer to that question, you need to look at the structure of an atom.

The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons.. Two or more atoms held together by. 14.05.2017 · this chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. In chemistry, there are different terms to describe the structure of matter, such as atom vs. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. To know the answer to that question, you need to look at the structure of an atom. In chemistry, there are two main types of particle that we need to know about. Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound; Contrary to this, the molecule comprises two or more than two atoms... The atom comprises of the …

Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound; Atom is defined as the smallest unit of an element which may or may not exists independently. 14.05.2017 · this chemistry video tutorial explains the difference between elements, atoms, molecules, and ions.. So, what is the difference …

The atom comprises of the … It also explains how to distinguish ionic and molecular. A molecule is a group of two or more atoms that represent the smallest identifiable unit of a pure substance and retains the composition and chemical properties of the substance.. Atoms may or may not exist in the free state, but molecules exist in the free state.
On the other hand, the molecule is the smallest fundamental unit of the compound that always exists in the free state. 14.05.2017 · this chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. In chemistry, there are two main types of particle that we need to know about. So, what is the difference … The atom comprises of the … 07.12.2018 · the difference between atom and molecule can be drawn clearly on the following grounds: But when it comes to an atom vs... An atom is the smallest unit of matter composed of a nucleus and one or more electrons present around the nucleus.

14.05.2017 · this chemistry video tutorial explains the difference between elements, atoms, molecules, and ions.. In chemistry, there are two main types of particle that we need to know about. The atom comprises of the … The atom is reactive as it is less stable as compared to the molecule. So, what is the difference … Atom is defined as the smallest unit of an element which may or may not exists independently.

Contrary to this, the molecule comprises two or more than two atoms... Molecule, you know the atom came first.

While there are other fun subatomic particle names thrown in there, those are the three... Now the only question is why? Contrary to this, the molecule comprises two or more than two atoms. Atom is defined as the smallest unit of an element which may or may not exists independently. Contrary to this, the molecule is less reactive and more stable. An atom is the smallest unit of matter. Molecule noun (chemistry) the smallest particle of a specific element or compound that retains the chemical properties of that element or compound; Atom may or may not exist in the free state while molecule always exists in the free state. A molecule is a group of two or more atoms that represent the smallest identifiable unit of a pure substance and retains the composition and chemical properties of the substance. It also explains how to distinguish ionic and molecular. To know the answer to that question, you need to look at the structure of an atom.. But when it comes to an atom vs.
